Can't stay dry no matter what you try?
Get Sofdra NowGet Sofdra for $0 copay with commercial insurance.*
For adults and children 9 years of age and older with excessive underarm sweating, also known as primary axillary hyperhidrosis.
*For patients with commercial insurance, such as employer‑sponsored plans. Additional Terms and Conditions apply.



Drier days start at bedtime
Sofdra starts working while you sleep to help keep you drier all day. Clinically proven to deliver next-day results.
See the results
Sweat control down to a science
Unlike antiperspirants that block pores, Sofdra targets the actual signal that tells your underarms to sweat in the first place.
See how it works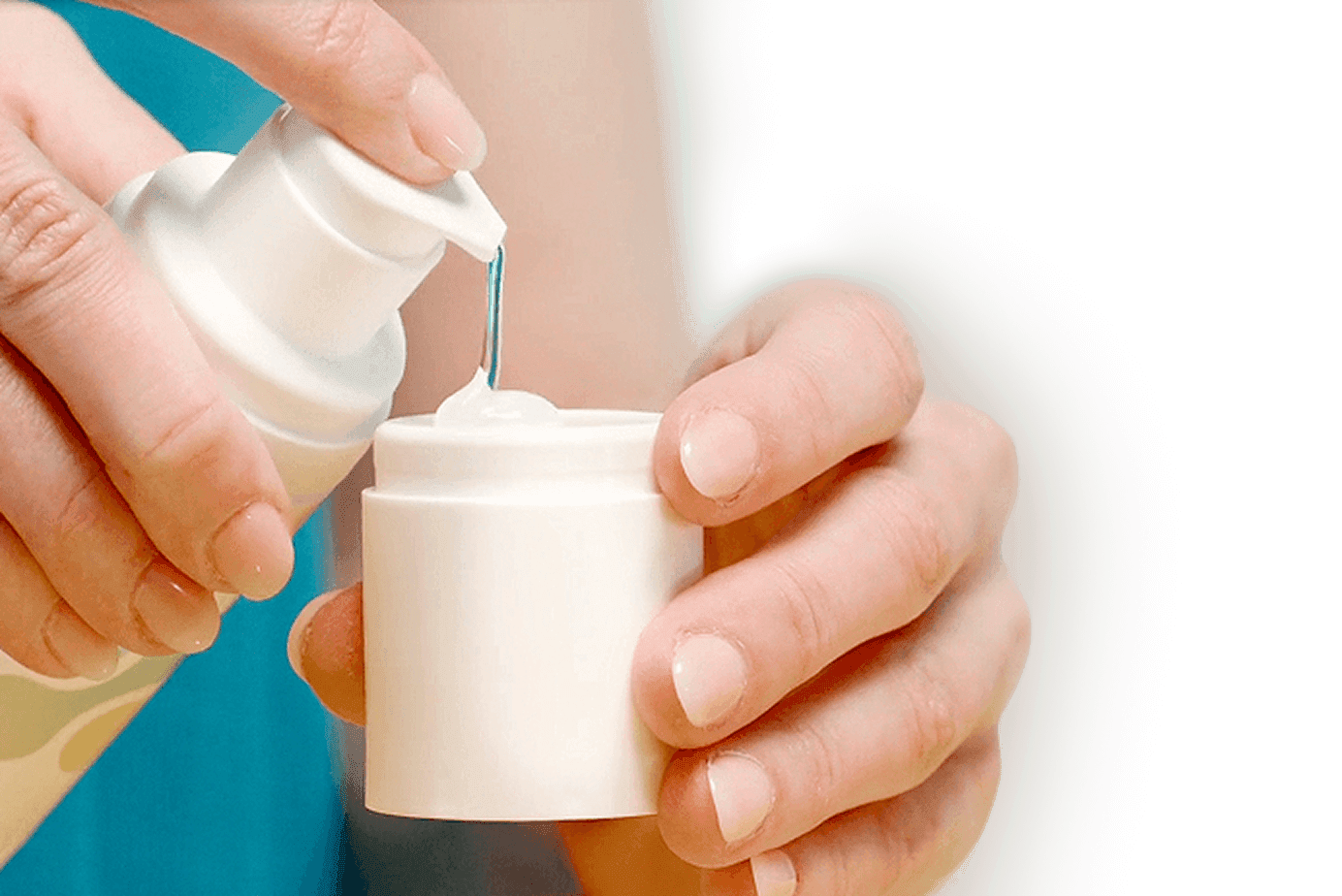
Designed for simplicity
Sofdra goes on smoothly with a built-in applicator that keeps things quick and clean. Just prepare, apply, and let it dry.
See how to applyNo doctor's office
visit needed!
Skip the waiting room. We'll ship Sofdra directly to your door—fast.
Start your online consult with a telehealth provider now to see if you are eligible.
Indication
Sofdra (sofpironium) topical gel, 12.45% is a prescription anticholinergic medicine used on the skin to treat excessive underarm sweating (primary axillary hyperhidrosis) in adults and children 9 years of age and older.
IMPORTANT SAFETY INFORMATION
Sofdra is for use on the skin in the underarm area only. After applying Sofdra, wash your hands right away and do not touch your underarms. Sofdra is flammable. Avoid heat and flame while applying Sofdra.
Who should not use Sofdra?
Do not use Sofdra if you have certain medical conditions that can be made worse by taking an anticholinergic medicine, such as glaucoma, severe ulcerative colitis (UC) or other serious bowel problems, myasthenia gravis, and Sjogren’s syndrome.
What should I tell my healthcare provider before using Sofdra?
- Tell your healthcare provider about all of your medical conditions, including prostate, bladder or kidney problems, problems passing urine, if you are pregnant or breastfeeding, or plan to become pregnant or breastfeed. It is not known if Sofdra will harm your unborn baby or pass into your breast milk.
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, especially any anticholinergic medicines.
What are possible side effects of Sofdra?
Serious side effects may include:
- Urinary retention. Stop using Sofdra and call your healthcare provider right away if you have difficulty urinating, urinate frequently or in a weak stream or drips, a full bladder or difficulty emptying your bladder.
- Problems with control of your body temperature. Stop using Sofdra and call your healthcare provider right away if you have decreased sweating that develops into symptoms of heat illness (e.g., hot, red skin; passing out; fast, weak pulse; fast, shallow breathing; increased body temperature).
- Blurred vision. Stop using Sofdra, call your healthcare provider right away, and do not drive or operate machinery or do hazardous work until your vision is clear.
The most common side effects of Sofdra include dry mouth; blurred vision; pain, redness, swelling, itching, and irritation in the underarm area; dilation of the pupils of your eyes; and problems with urination.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1‑800‑FDA‑1088. You may also report side effects to Botanix at 1‑866‑763‑6337.
Keep Sofdra and all medicines out of the reach of children.
Please see full Prescribing Information, Patient Product Information, and Instructions for Use.